Democratizing Genomic Health for Every Family
Nature | 8% of Stomach Cells After Age 60 Carry Cancer-Related Mutations! Scientists Uncover Key Mechanisms of Precancerous Mutations in Gastric Cancer
3/27/20253 min read


Recently, a study led by the Wellcome Sanger Institute and the University of Hong Kong, published in Nature, has systematically revealed the genetic mutation accumulation patterns preceding gastric cancer. By mapping the somatic mutation landscape of normal gastric epithelial samples from different parts of the gastrointestinal tract, the researchers found that gastric epithelial cells accumulate driver gene mutations with age and chronic inflammation, even in individuals without cancer. Notably, approximately 8% of gastric epithelial cells in individuals over 60 years old already carry cancer-related genetic alterations.
Multi-Omics Analysis Reveals the Panoramic Mutational Landscape of the Stomach, Hiding Cancer-Related Codes in Normal Tissue
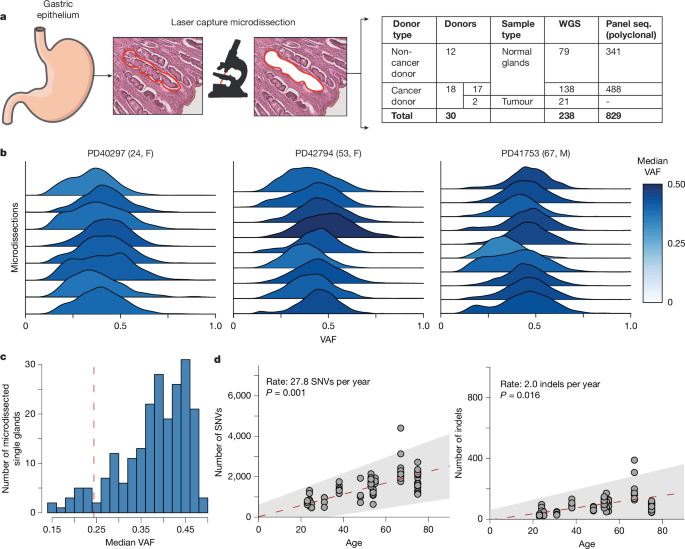
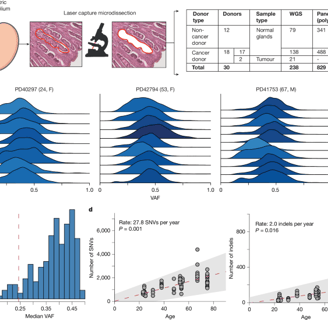
The research team used laser microdissection combined with whole-genome sequencing to analyze 238 microdissected samples from four gastrointestinal sites in 30 donors (including 18 gastric cancer patients) and performed targeted sequencing of cancer driver genes on 829 gastric lobule samples. The results showed:
Mutation Rate Differences: Normal stomach/esophageal tissue accumulates an average of 30 single-nucleotide mutations per year, while the small intestine/colon accumulates 50.
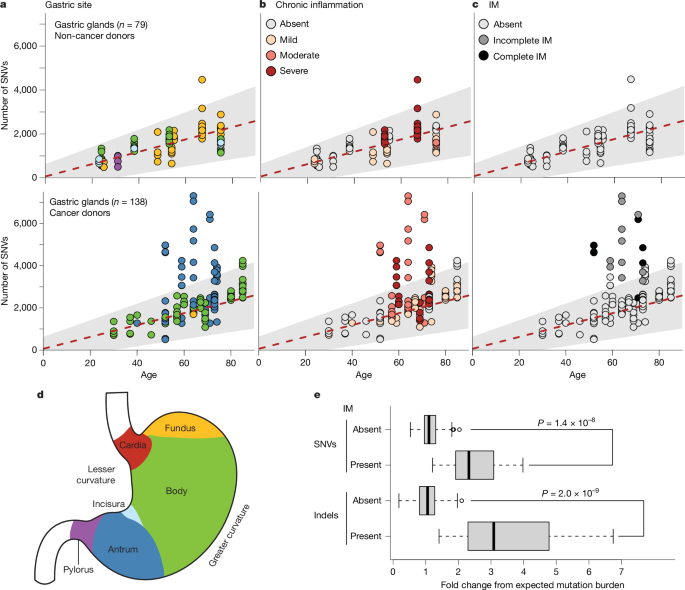
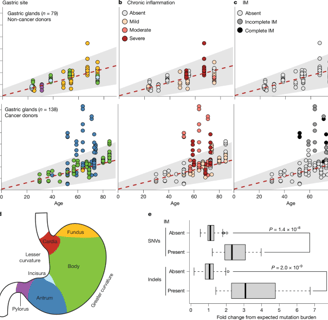
Inflammation Accelerates Carcinogenesis: Gastric tissue in gastric cancer patients showed significantly higher mutation burdens, closely linked to oxidative damage and cell proliferation.
High-Risk Signal Identification: Key driver gene mutations (e.g., ARID1A, CTNNB1, KDM6A) were frequently detected in regions of chronic inflammation.
"For years, we have recognized that precancerous clones are common in normal tissues, yet overt cancer is relatively rare," explained the lead author, Tim Coorens. "Understanding the pathways by which normal cells transition to cancer cells—through mutations, environmental stress, or other mechanisms—enables early cancer detection, risk factor identification, and potential prevention."
Clonality and Mutation Rates: Chronic Inflammation as a "Catalyst" for Carcinogenesis
"Chronic inflammation not only selects for cell clones carrying driver mutations but also induces intestinal metaplasia in gastric glands—a lesion that dramatically increases mutation burden and significantly elevates cancer risk," Coorens noted. However, abnormal changes are not limited to cancerous or precancerous cells. Clones of cells with driver gene alterations appear to increase with chronic inflammation and age, accounting for approximately 8% of gastric epithelial lining cells in participants aged 60 and older.
Gastric Segments, Chronic Inflammation, and Metaplasia
Additionally, the research team detected frequent trisomy of chromosomes 13/20 in seemingly healthy gastric epithelial samples. This specific aneuploidy (three copies of a chromosome) is hypothesized to arise from genomic instability triggered by early infection history or specific environmental exposures. "We have not observed this in any other tissue, suggesting an unknown external carcinogen that may have been encountered by only a subset of individuals," noted Professor Snow Chan, co-senior author from the Department of Pathology at the University of Hong Kong and the Hong Kong Science Park Cancer Immunology Research Centre.
Uncovering Precancerous Features and Building Early-Screening Prediction Models
Although the pathogenic mechanisms of trisomy and its exact association with gastric cancer development require further investigation, this study is the first to demonstrate through large-scale sequencing that systematic analysis of somatic mutation patterns can reveal precancerous progression features that are difficult to identify through traditional pathology.
The research team is applying these findings to the U.S. National Institutes of Health (NIH)-funded "Somatic Mosaicism in Human Tissues" (SMaHT) initiative. "With the maturation of high-throughput sequencing technologies, we can now systematically map the mutational evolutionary trajectories in normal tissues," Coorens said. This international project plans to perform deep sequencing on normal tissue samples from diverse ethnic populations to establish predictive models for early cancer progression